

In cases like these, how do we evaluate the relative importance of each resonance form? 4. Take the ketone below (acetone, or “propanone”) for which we can draw 3 different resonance forms. In the case of the acetate ion and the allyl cation, both resonance forms are equal in energy, so the “hybrid” is a 1:1 mixture of the two. So How Do We Evaluate How “Important” Each One Is? Not All Resonance Forms Are Of Equal Significance. Instead, the “true” state of the molecule will be a “hybrid” of these resonance forms.įor example in the acetate and allyl cation examples below, the “true” structure of the molecule is represented through a 50:50 combination of the two resonance forms.ģ. It is tempting (and very wrong!) to think that these resonance forms are in “equilibrium” between each other. Here’s an important point about resonance forms. Remember That Resonance Structures Are Not In Equilibrium With Each Other – They Represent Contributions To An Overall Resonance “Hybrid” There are three “legal” ways to move electrons using curved arrows: from pi bond to lone pair, from lone pair to pi bond, and from pi bond to pi bond:Ģ. How can we “find” resonance forms for a given molecule? It’s possible to do it through trial-and-error, but one surefire way is to do so is to apply the curved arrow formalism, which is a way of depicting the “movement” of electrons.
Therefore, in order to understand electron density on a molecule where pi bonds are present, we must first understand the importance of its various resonance forms. However if multiple bonds (π bonds) are present, then we start to run into a little problem: there can be multiple ways to distribute electrons on the same molecule (i.e. One of the key skills in analyzing the reactivity of a molecule is to be able to figure out where the electrons are.Īs I wrote here, if we’re dealing with single bonds, it’s a relatively straightforward matter of figuring out the differences in electronegativities. Recall The Three “Legal” Electron-Pushing Arrow “Moves” Used For Interconverting Resonance StructuresĪfter all these posts about resonance, I thought it would be good to have a post summarizing what’s been discussed so far.
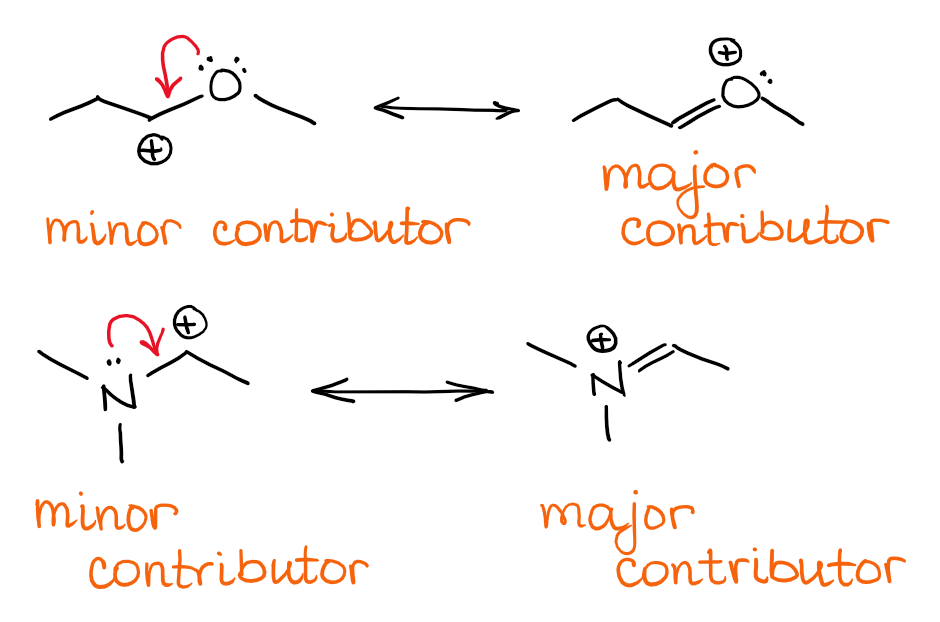
#Explain resonance in chemistry full
Rule #2: Full Octets Are Preferable To Empty Octets (And Never, Ever Have Empty Octets On Oxygen or Nitrogen!).Rule #1: Neutral Resonance Structures Are More “Important” Than Charged Resonance Structures.Not All Resonance Forms Are Of Equal Significance.Remember That Resonance Structures Are Not In Equilibrium With Each Other – They Represent Contributions To An Overall Resonance “Hybrid”.Recall The Three “Legal” Electron-Pushing Arrow “Moves” Used For Interconverting Resonance Structures.
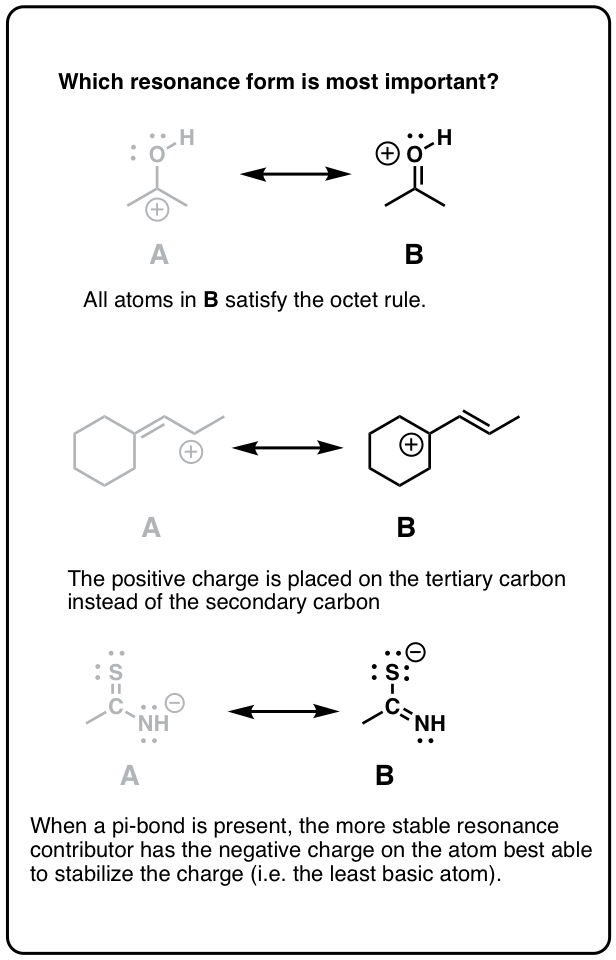
Today, let’s summarize everything we’ve learned about resonance structures in this unit.


 0 kommentar(er)
0 kommentar(er)
